New Narcolepsy Medication 2024 – Harmony Biosciences Holdings, Inc. (Nasdaq: HRMY) has announced that the U.S. Food and Drug Administration (FDA) has granted priority review to its supplemental New Drug Application (sNDA) for WAKIX® . The Global Narcolepsy Treatment Industry is on the verge of a substantial breakthrough, with Future Market Insights (FMI) forecasting a remarkable growth trajectory. From an estimated US$4.77 billion .
New Narcolepsy Medication 2024
Source : www.cnn.comADHD, narcolepsy medication recalled as antihistamine pills might
Source : www.mlive.comRecall: Children’s ADHD/Adult Narcolepsy Medication Mix up
Source : www.medwastemngmt.comFor Narcolepsy Patients, Drug Shortage and Stigma Restrict Care
Source : undark.orgADHD drug Zenzedi voluntarily recalled after manufacturing error
Source : www.newsnationnow.comBig Pharma CNS Acquisition, Precision Psychiatry Raise, Genomic
Source : www.linkedin.comAdderall Alternative 2024 Discover Safe And Legal Over The
Source : www.ndtv.comPatients with narcolepsy face a dual nightmare of medication
Source : health.wusf.usf.eduRegistration for 2024 Wake Up Narcolepsy National Summit is NOW
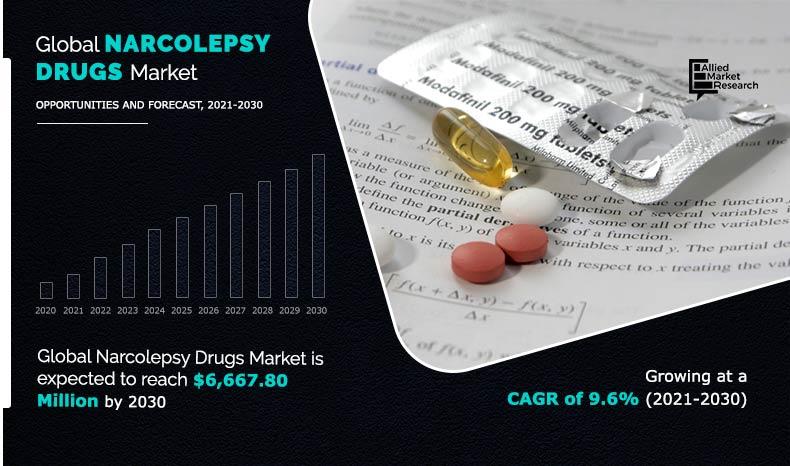
Source : www.wakeupnarcolepsy.orgNarcolepsy Drugs Market Size, Share, Trends Analysis 2021 2030
Source : www.alliedmarketresearch.comNew Narcolepsy Medication 2024 Zenzedi: ADHD medication recalled due to pill mixup | CNN: Bedtime drug being developed by Tonix relieved pain by targeting fibromyalgia’s characteristic “non-restorative sleep”: Potential for FDA Approval in 2025 CHATHAM, NJ / ACCESSWIRE / / People who are . A drugmaker’s feud with the DEA is exacerbating the ADHD meds crisis — at a rate of 600 million missing doses a year. .
]]>